By Nicole KwanPublished May 15, 2014FoxNews.com
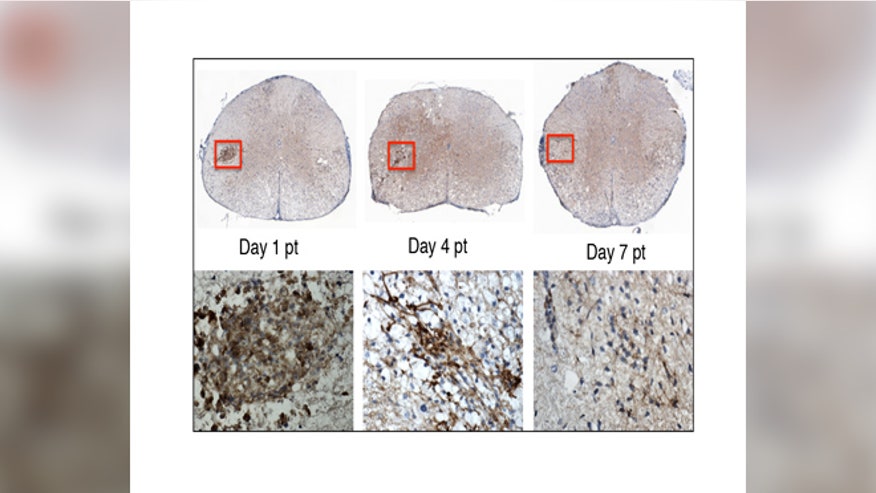
In this image, the top row shows the stem cells transplanted into the mouse spinal cord. The lower row shows a close-up of the stem cells (brown). By day 7 post-transplant, the stem cells are no longer detectable. Within this short period of time, the stem cells have sent chemical signals to the mouse’s own cells, enabling them to repair the nerve damage caused by MS. (image: Lu Chen)
or patients with multiple sclerosis (MS), current treatment options only address early-stage symptoms of the debilitating disease. Now, new research has found a potential treatment that could both stop disease progression and repair existing damage.
In a study published in Stem Cell Reports, researchers utilized a group of paralyzed mice genetically engineered to have an MS-like condition. Initially, the researchers set out to study the mechanisms of stem cell rejection in the mice. However, two weeks after injecting the mice with human neural stem cells, the researchers made the unexpected discovery that the mice had regained their ability to walk.
“This had a lot of luck to do with it; right place, right time” co-senior author Jeanne Loring, director of the Center for Regenerative Medicine at The Scripps Research Institute in La Jolla, California, told FoxNews.com. “[co-senior author Tom Lane] called me up and said, ‘You’re not going to believe this.’ He sent me a video, and it showed the mice running around the cages. I said, ‘Are you sure these are the same mice?’”
Loring, whose lab specializes in turning human stem cells into neural precursor cells, or pluripotent cells, collaborated with Tom Lane, a professor of pathology at the University of Utah whose focus is on neuroinflammatory diseases of the central nervous system. The team was interested in stem cell rejection in MS models in order to understand the underlying molecular and cellular mechanisms contributing to rejection of potential stem cell therapies for the disease.
Multiple sclerosis is an autoimmune disease that affects more than 2.3 million people worldwide. For people with MS, the immune system misguidedly attacks the body’s myelin, the insulating coating on nerve fibers.
“In a nutshell, it’s the rubber sheath that protects the electrical wire; the axon that extends from the nerve’s cell body is insulated by myelin,” Lane, who began the study while at the University of California, Irvine, told FoxNews.com
Once the myelin has been lost, nerve fibers are unable to transmit electric signals efficiently, leading to symptoms such as vision and motor skill problems, fatigue, slurred speech, memory difficulties and depression.
The researchers’ inadvertent treatment appeared to work in two ways. First, there was a decrease of inflammation within the central nervous system of the mice, preventing the disease from progressing. Secondly, the injected cells released proteins that signaled cells to regenerate myelin and repair existing damage.
While the stem cells were rejected in the mice after 10 days, researchers were able to see improvements for up to six months after initial implantation.
“One of the big hurdles in stem cell transplant therapies is rejection. What our data would argue, [is immunosuppression] may be a moot point because it doesn’t matter if [stem cells] are rejected, because they are, but we still see clinical improvement,” Lane said.
Now the team is working on figuring out how to use the stem cell-secreted proteins, or something that mimics the proteins, for clinical use— instead of transplanting stem cells directly, which would be more difficult both in creation and practical application. Additionally, drugs they create could get FDA approval more easily, compared to stem cell therapy, Loring said.
“Rather than delivering stem cells for MS, we want to deliver things stems cells make that are good for mice and presumably humans,” Loring said.
CONTINUE reading this stem cell therapy update
……..
…….
Share our Articles with others
Stay informed with MS news and information - Sign-up here
For MS patients, caregivers or clinicians, Care to chat about MS? Join Our online COMMUNITY CHAT




