======================================
REPAIR, REGENERATE & RENEW: Cutting-Edge Stem Cell Research at Tisch MS Research Center of New York
REPAIR

REGENERATE
In 2007, we started the process of obtaining FDA approval for a Phase I clinical trial. Finally, in August 2013, we received FDA approval to move ahead!
- The objective of the Phase I trial is to test safety.
- Data from the Phase I trial will lead to an improved strategy for Phase II
- Positive outcomes of the trial may indicate the use of this therapy in other neurological diseases like ALS and Parkinson’s Disease, cerebral palsy and spinal cord injuries.

Why Stem Cells?
In pre-clincal, non-human experiments of our stem cell therapy, renewal, repair and regeneration of myelin occurred in the CNS after intrathecal (via spinal fluid, not intravenous) injection.From our preclinical models, we know that stem cells injected into the spinal fluid can have significant benefit in terms of improved neurological symptoms.
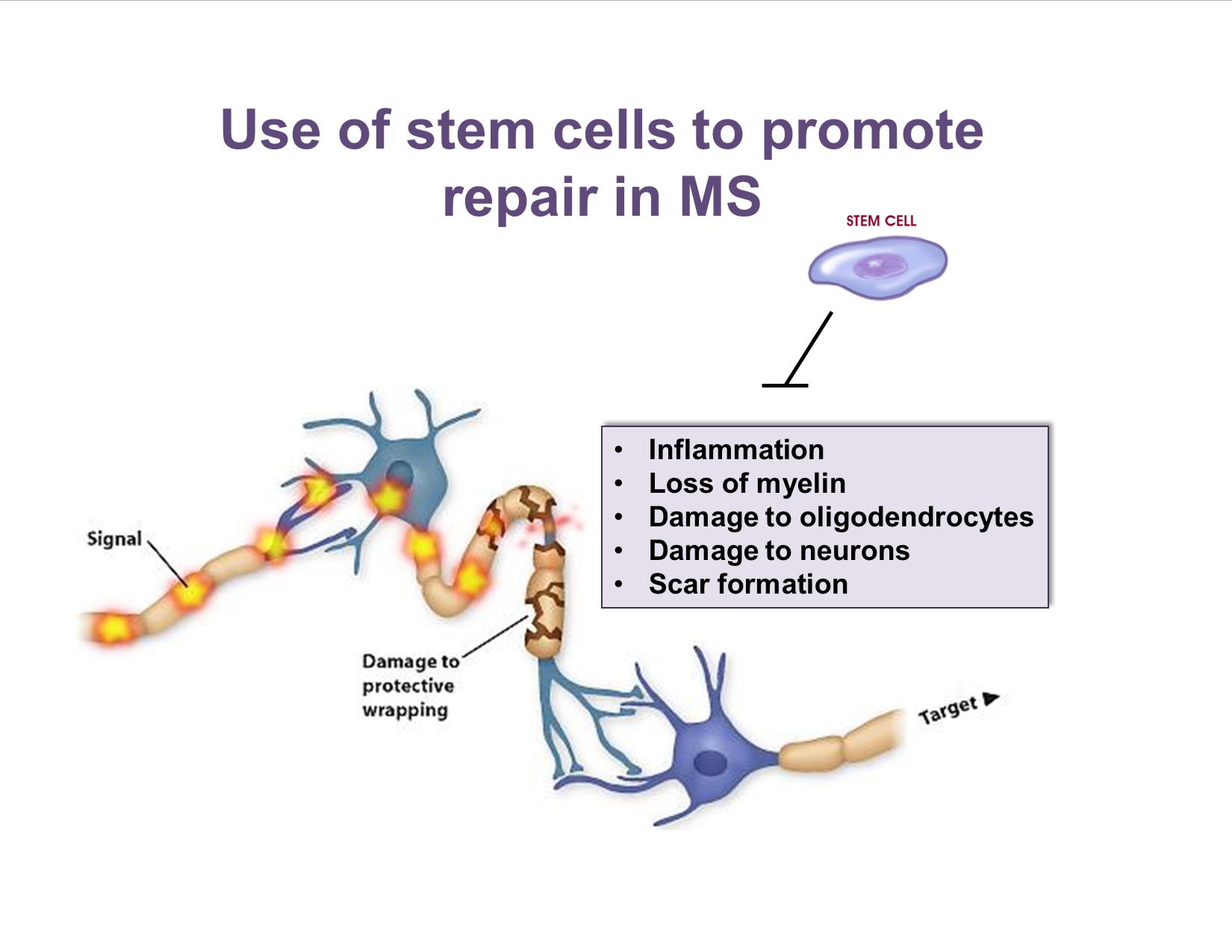
MS is characterized by brain inflammation, loss of myelin, damage to oligodendrocytes (the cells that make myelin), damage to neurons, and scar formation in the brain. As a result, nerve signals are not properly conducted, causing loss of neurological function. Stem cells are hypothesized to promote repair in MS by migrating to areas of demyelination, blocking damage-forming events, and enabling repair.
Why Neural Stem Cells?
WATCH OUR MS EDUCATIONAL VIDEOS by Topic, found here: www.youtube.com/msviewsandnews
JOIN our Facebook Page: www.facebook.com/msviewsandnews
Stay informed with MS news and information - Sign-up here
For MS patients, caregivers or clinicians, Care to chat about MS? Join Our online COMMUNITY CHAT




