Medscape Medical News
October 18, 2010 (Gothenburg, Sweden) — A new assay measuring JC virus antibodies is in development and could be available as early as January. Biogen Idec and Elan Corporation are racing to bring a new tool to market that they hope will boost confidence in natalizumab (Tysabri) for multiple sclerosis (MS).
Allison Gandey
October 18, 2010 (Gothenburg, Sweden) — A new assay measuring JC virus antibodies is in development and could be available as early as January. Biogen Idec and Elan Corporation are racing to bring a new tool to market that they hope will boost confidence in natalizumab (Tysabri) for multiple sclerosis (MS).
Large-scale, prospective clinical studies are currently under way to determine whether a new JC virus assay will help clinicians predict which patients are most at risk for progressive multifocal leukoencephalopathy (PML).
Presenting here at the 26th Congress of the European Committee for Treatment and Research in Multiple Sclerosis, Al Sandrock, MD, Biogen’s seThe false-negative rate for the new test is about 2.5%. The spontaneous seroconversion rate of negative-testing patients is estimated at about 2% per year. Both suggest that periodical testing might be warranted in patients taking natalizumab in clinical routine.
nior vice The false-negative rate for the new test is about 2.5%. The spontaneous seroconversion rate of negative-testing patients is estimated at about 2% per year. Both suggest that periodical testing might be warranted in patients taking natalizumab in clinical routine.
president of neurology research and development in Cambridge, Massachusetts, described the project.
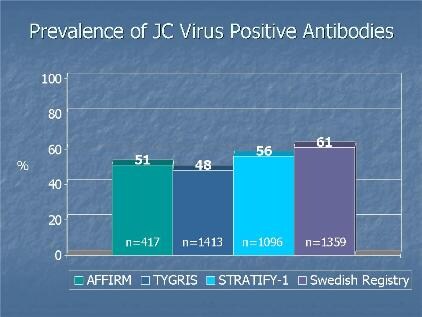 |
| Antibodies consistent across studies. |
The 2-step enzyme-linked immunosorbent assay screens and confirms the presence of JC virus antibodies. So far, the test has been evaluated in more than 5000 patients.
Most of the people are from 4 large natalizumab studies. AFFIRM is a phase 3 multicenter international trial. TYGRIS is a US and Canadian observational study. STRATIFY-1 was launched as an anti–JC virus antibody study for patients receiving or considering natalizumab. And the final study uses data from a Swedish MS registry.
Dr. Sandrock reports the incidence of PML is lower in anti–JC virus–negative compared with anti–JC virus–positive patients. The prevalence of antibodies against JC virus was consistent across studies and ranged from 48% to 61%.
Dr. Sandrock suggests a PML global incidence of 1.63 per 1000 patients treated with natalizumab for at least 18 months. He says the prevalence of antibodies was not affected by prior immunosuppressant use. The virus was less often found in women, but the likelihood of testing positive seemed to increase with age.
False Negatives
The false-negative rate for the new test is about 2.5%. The spontaneous seroconversion rate of negative-testing patients is estimated at about 2% per year. Both suggest that periodical testing might be warranted in patients taking natalizumab in clinical routine.
***********************************************************
“Providing You with ‘MS Views and News’, is what we do“
Keep Informed and up–to–date with information concerning
Multiple Sclerosis when registered at
the MS Views and News website.
****************************************************************
Disclaimer: ‘MS Views and News’ (MSVN), does not endorse any products or services found on this blog. It is up to you to seek advice from your healthcare provider. The intent of this blog is to provide information on various medical conditions, medications, treatments, and procedures for your personal knowledge and to keep you informed of current health-related issues. It is not intended to be complete or exhaustive, nor is it a substitute for the advice of your physician. Should you or your family members have any specific medical problem, seek medical care promptly.
. ****************************************************************
Visit our MS Learning Channel on YouTube: http://www.youtube.com/msviewsandnews


