Medical Need Europe, a privately held pharmaceutical company headquartered in Sweden and focused on registration, distribution, marketing and sale of orphan drugs and niche speciality pharmaceuticals for treatment of rare diseases, has appointed London, U.K. based Durbin PLC, a leading global supplier and distributor of pharmaceuticals, to manage warehousing and supply chain for a range of Medical Need products.
At the outset, this collaboration will involve a single product, Medical Need’s progressive multiple sclerosis (MS) management drug MD1003, which is currently under clinical development.
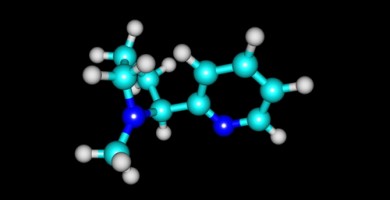
In April, Medical Need partner MedDay Pharmaceuticals, a biotechnology company focused on treatment of nervous system disorders, founded in 2011 by neurologist and neuroscientist Fredric Sedel, MD, PhD, and Guillaume Brion, announced positive results from the pivotal randomized 2:1, double-blinded, placebo-controlled Phase III clinical trial MS-SPI. The trial was conducted in 16 MS reference centers in France, and showed evidence of the efficacy and safety of MD1003, a highly concentrated pharmaceutical-grade biotin administered at a dose of 300 mg per day for treatment of primary and secondary progressive multiple sclerosis, a major area of unmet medical need. Treatment duration was one year.
The company reported that the MS-SPI clinical trial’s primary endpoint was met with a proportion of MS patients showing an improvement in EDSS (expanded disability status scale) or TW25 (timed 25-foot walk) at Month 9 and confirmed at Month 12 (p = 0.0051). The primary endpoint was supported by evidence of a substantial decrease in the risk of disease progression.
The patient population was defined as patients suffering from primary progressive Multiple Sclerosis (PPMS) or secondary progressive Multiple Sclerosis (SPMS) with EDSS progression within the two years prior to inclusion, and with EDSS ranging from 4.5 to 7. Patients with disease modifying therapy (DMT) introduced less than 3 months prior to inclusion; fampridine introduced less than 1 month prior to inclusion; or evidence of relapse or Gadolinium-MRI activity within the past year were excluded from the study.
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
MS Views and News helps to provide information for all affected by MS
Keep up to date with the news and information we provide
by signing up by clicking here
.===================================
Visit our MS Learning Channel on YouTube: http://www.youtube.com/msviewsandnews