sclerosis is a particularly horrendous and intractable illness. Unlike the
relapsing remitting form of the disease, for which there are currently 12
approved treatment options (however imperfect these may be), there is
tragically little available for progressive MS patients (one very flawed
treatment option for SPMS and none for PPMS). At the recent American Academy of
Neurology meetings, held last week in Washington DC, some rays of hope for
progressive MS finally shone through, among them studies done on
honest-to-goodness human progressive MS patients – as opposed to those done on
mice or in test tubes – that show particular promise.
As I’ve written about extensively (click here ), the Tisch MS Research Center of New York is
currently conducting the only FDA approved regenerative human stem cell trial
on MS patients in the United States. Yes, this is the very same study that the
National Multiple Sclerosis Society has repeatedly refused to fund (click here). Though this phase 1 trial is not yet
complete, interim results were released at the AAN meeting, and they look
impressive.
The Tisch Center utilizes a unique approach to using stem cells to treat MS,
quite unlike the techniques used in previous regenerative stem cell trials or
the stem cell treatments being offered by for-profit operations scattered
around the world. Employing proprietary methodology developed in the Tisch
Center’s research laboratories, raw mesenchymal stem cells – harvested from
each patient’s bone marrow – are transformed into stem cells specific to the
human nervous system, called neural progenitor cells. The 20 patients enrolled
in this early stage trial will each receive three spinal (intrathecal)
injections of neural progenitor cells, spaced three months apart. The interim
results released last week report on the nine patients who have thus far begun
treatment (click here).
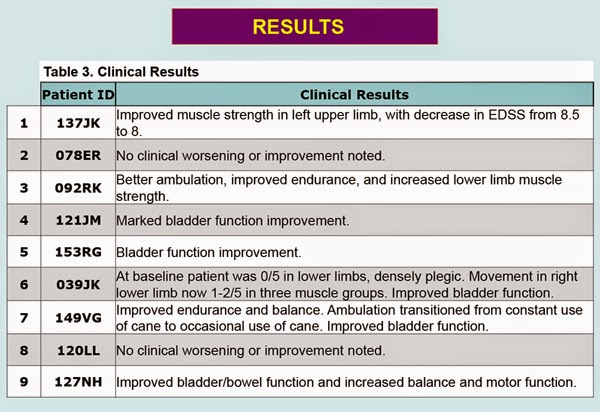
Of these nine patients, seven displayed some form of disease improvement. Six
of these seven patients suffer from SPMS, and one from PPMS. Based on
neurologic exams, five of these seven patients displayed improved motor
functions, including better balance, increase muscle strength, and improved
ambulation. Six of the patients reported better bladder function. No
significant adverse events were reported. Here’s a graphic detailing the Tisch
Center stem cell trial results on a patient by patient basis, taken from the
poster presented at the AAN meetings. To view the full poster, please (click here).
While exciting, it’s important to keep these results in perspective. We’re
looking at a very small patient population taking part in an early phase 1
trial whose primary endpoint is establishing the safety of the treatment. That
said, given the intractability of progressive MS, seeing any significant
improvement is extremely encouraging, and these early results certainly
validate the approach to regenerative stem cell therapy being taken by the
Tisch Center.
Alarmingly, though, the Tisch Center is now facing a fund-raising crisis that
threatens to impede the phase 2 extension of this study, as well as much of the
other groundbreaking MS research currently underway in the Tisch laboratories.
In previous posts I’ve expressed my extreme distress at the NMSS’s repeated
refusals to fund research being done at the Tisch Center, and due to unforeseen
circumstances the Center’s funding shortfall is now being felt quite acutely.
The animal research laboratory used by Tisch Center scientists is being closed
as a result of the sale of the hospital in which it’s located (only a block
away from the Tisch MS Center), leaving the Center with no viable alternative
other than constructing their own facility, which will require a massive
fund-raising effort.
Since I’d rather this post concentrate on the research itself, I urge all
readers to click here for more information regarding this
fund-raising crunch, and to spread the word far and wide. While the Tisch
Center is actively conducting the only current FDA approved MS stem cell trial
on human beings, the NMSS funds preclinical stem cell experiments being done in
test tubes and on mice that, even if spectacularly successful, won’t reach MS
patients for more than a decade. Just saying…
(Full disclosure: I am a patient at the MS clinic directly associated with
the Tisch MS Research Center of New York, and my MS as well as other physical
ailments have been totally kicking my ass lately. So, yeah, I might take this
crap just a wee bit personally.)
Another much-anticipated study presented at last week’s AAN conference provided
yet more hope for progressive MS patients, though perhaps not as much as
originally anticipated. The French pharmaceutical company MedDay released the
results of a stage III clinical trial involving the use of massive doses of
Biotin to treat patients with Primary Progressive Multiple Sclerosis (PPMS) and
Secondary Progressive Multiple Sclerosis (SPMS).
Biotin (vitamin B7, also known As Vitamin H or Coenzyme R) has been used in
much lower doses as an over-the-counter “nutraceutical” supplement to treat
brittle hair and nails, some skin conditions, and neuropathy brought on by type
II diabetes, among other applications (click here). Biotin is known to be necessary for cell
growth, the production of fatty acids, and the metabolism of fats and amino
acids (click here).
A small pilot study researching the use of high doses of Biotin to treat MS was
conducted by MedDay starting in 2013. This initial study produced astounding
results, with 91.3% of the 23 progressive MS patients involved displaying
improvements in their neurologic condition (click here). This small, unblinded, non-placebo-controlled
trial created much excitement, leaving patients and researchers awaiting the
results of a much larger placebo-controlled phase 3 trial, which was completed
in late 2014. The results of this phase 3 trial were presented at the AAN
conferences on Friday, April 24, 2015.
In this late stage study, conducted at 19 centers around France, patients were
given 300 mg of Biotin per day, which is the equivalent of approximately 10,000
times the maximum daily recommended dosage. The study involved 154 patients,
103 given Biotin and 51 given a placebo. The results of this study (click here), while positive, don’t appear to be nearly as
compelling as had been anticipated based on the early pilot study results.
The results of MedDay’s late stage study revealed that after 12 months, 12.3%
of the Biotin treated patients displayed a verified improvement in disability
scores, as opposed to 0% of the placebo group. Secondarily, 4% of Biotin
treated patients displayed disease progression after one year, versus 13% in
the placebo group. Very few adverse events, all considered non-serious, were
reported. While these numbers pale in comparison with those seen in the initial
pilot study, they still represent a breakthrough of sorts in treating advanced
progressive MS, which thus far has defied almost all attempts at treatment.
Looked at another way, about 1 in 8 patients treated with Biotin saw their
disability scores improve, while 1 in 25 saw their disease progress. Interestingly,
only 1 in 8 (I’m using ballpark figures here) untreated patients experienced
disease progression. While these aren’t the kind of results many hoped for
based on the early Biotin study results (9 in 10 patients experiencing
neurologic improvement), they are still better than nothing, which is what
mainstream medicine currently offers patients with advanced (non-relapsing)
progressive MS.
Given these factors, many patients with progressive MS (myself included) have
expressed great interest in giving Biotin a try, especially since the stuff is
readily available in over-the-counter form. The highest dose capsules
commercially available are 10 mg, meaning that a patient would need to take 30
capsules a day to replicate the doses used in MedDay’s trials, which
administered 100 mg of biotin three times a day. There are a few serious
problems with this approach, though, above and beyond the huge amount of
capsules that would need to be ingested to replicate the doses used in MedDay’s
trial.
First, the compound used in the MedDay trial is a highly concentrated and
purified pharmaceutical grade form of Biotin called D-Biotin, a stereoisomer of
Biotin that is extremely bioavailable (easily absorbed by the body) and
contains active enzymes (click here). This type of Biotin is generally not
available in over-the-counter capsules. Second, over-the-counter nutraceuticals
are completely unregulated, and it’s almost impossible to know the purity of
the compounds contained within the capsules or what other ingredients might
also be present. One study found that a shocking one third of herbal
supplements tested contained not a trace of primary ingredient the listed on
the bottle (click here)! Additionally, some Biotin supplements contain
calcium, which if taken in greater amounts can cause hypercalcemia, a
potentially very serious medical condition (click here).
After consulting with a naturopathic physician, I’m looking into procuring
ultra high grade, USP certified (click here)
D-Biotin from a reputable wholesaler and having it put into properly dosed
capsules through a compounding pharmacy (I’m doing this with my naturopath’s
help, of course, and will need a prescription in order to get the drug). While
this approach is likely to be much more expensive than using over-the-counter
product (probably about $300-$400/month), it will offer the best chance at
replicating MedDay’s trials, and would certainly eliminate the vast
uncertainties involved in consuming huge quantities of over-the-counter
nutraceutical supplements.
So, there you have it, two clinical trials targeting progressive MS in very
different ways, but coming up with encouraging results to one degree or
another. While the Tisch Center stem cell therapy is still in early trials and
is at least several years away from moving from the experimental stage to
general clinical use, MedDay’s Biotin compound should be ready for FDA approval
by the end of this year, and highly motivated patients might try to get a head
start on things by taking matters into their own hands. Although the results of
MedDay’s late stage phase 3 trial were a bit underwhelming, they do represent
an important advance over the status quo, and many progressive MS patients are
well past the “any port in a storm” stage.
.