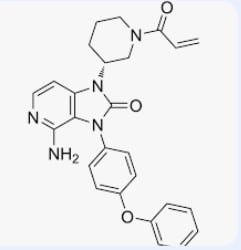
The BTK inhibitor tolebrutinib meets its primary endpoint in phase 3 HERCULES trial

For the first time, a medication has been shown to delay confirmed disability progression in individuals with nonrelapsing secondary progressive multiple sclerosis (MS).
The findings involve the investigational oral BTK inhibitor tolebrutinib and come from the phase 3 HERCULES study, whose results were shared in a platform presentation at the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) meeting on Sept. 20 by Cleveland Clinic neurologist Robert Fox, MD, chair of the study’s global steering committee.
“In delaying onset of confirmed disability progression by 31% relative to placebo, tolebrutinib met the HERCULES trial’s primary endpoint,” says Dr. Fox, staff in the Mellen Center for Multiple Sclerosis Treatment and Research and Vice Chair for Research in Cleveland Clinic’s Neurological Institute. “This represents very real progress on the path to effective therapy to delay disability progression in patients with secondary progressive MS, a population with substantial unmet need for effective therapies.”
Stay informed with MS news and information - Sign-up here
For MS patients, caregivers or clinicians, Care to chat about MS? Join Our online COMMUNITY CHAT



