August 20, 2021 – Marco Meglio
NeurologyLive, August 2021, Volume 4, Issue 4
The agent’s safety profile has been studied in more than 1200 individuals to date across several inflammatory diseases, with data indicating that the high selectivity of fenebrutinib may limit off-target effects.

Alexandra Goodyear, MD, MS
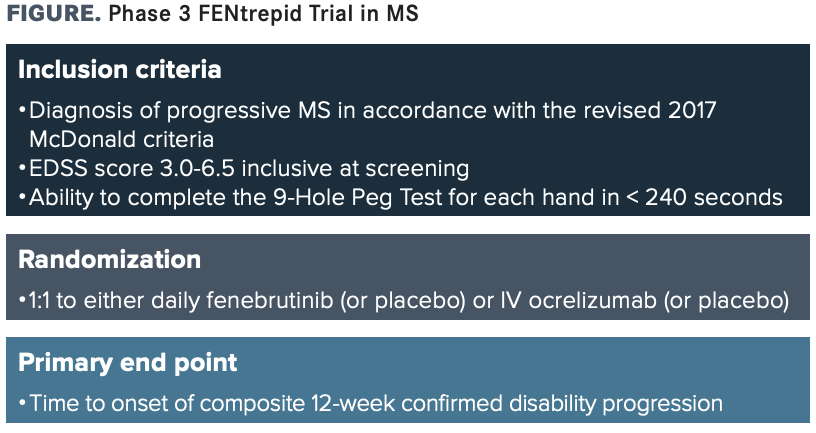
IN SEPTEMBER 2020, Roche announced it was initiating a new phase 3 clinical trial program for fenebrutinib, an investigational oral Bruton tyrosine kinase (BTK) inhibitor for the treatment of relapsing multiple sclerosis (RMS) and primary progressive MS (PPMS). The program included 2 identical phase 3 trials in RMS, FENhance 1 (NCT04586023) and FENhance 2 (NCT04586010), and a separate phase 3 trial in PPMS, FENtrepid (NCT04544449).1
FENtrepid, a multicenter, double-blind, double-dummy, parallel-group study, is expected to enroll up to 946 patients with PPMS aged 18 to 65 years. Eligible participants will be randomly assigned 1:1 to either daily oral fenebrutinib or placebo or intravenous ocrelizumab (Ocrevus; Genentech) for 120 weeks (FIGURE). An open-label extension trial may follow FENtrepid, depending on the observed benefits.
Investigators will use time to onset of composite 12-week confirmed disability progression (CDP) as the primary outcome measure, whereas time to onset of composite 24-week CDP, percentage change in total brain volume assessed by MRI, and change from baseline in participant-reported physical impacts of MS will all be among secondary end points observed.
Alexandra Goodyear, MD, MS, global development lead, Roche, told NeurologyLive®, “With a BTK inhibitor acting on the myeloid cells, we’re hopeful that we can act on the chronic inflammatory processes that seem to be underlying disability progression. Disability progression remains the key unmet need within the MS community.”
“It’s the dual mechanism of action that is really exciting. People have asked, ‘How do we address the compartmentalized inflammation that is pointing to underlying disability progression?’ BTK inhibitors are hitting all the B cells and myeloid cells that are acting on innate immunity.”
Fenebrutinib is unique because it is a noncovalent and reversible BTK inhibitor compared with other agents, such as ibrutinib (Imbruvica; AbbVie/Janssen), tolebrutinib (Sanofi), and evobrutinib (EMD Serono), which are all covalent and irreversible.
EDSS, Expanded Disability Status Scale; MS, multiple sclerosis.
Early clinical safety data thus far have confirmed the high selectivity of fenebrutinib compared with other BTK inhibitors. The reasons for its high selectivity stem from its binding mode; the agent binds orthogonally and selects a unique pocket. In whole blood assays, fenebrutinib potently inhibits myeloid and B-cell activation, shown by half-maximal inhibitory concentrations of 8 nM and 31 nM, respectively.2
The rate at which a drug dissociates from its target can influence its duration of action and efficacy. Data also showed that although fenebrutinib’s residence time in vivo may be less than 18 hours, its slow dissociation from BTK may positively influence efficacy. Investigators also concluded that its preclinical pharmacokinetic characteristics are favorable, indicating the potential for once-daily oral dosing.
A group of investigators, including Goodyear, presented research at MSVirtual2020, the 8th Joint ECTRIMS-ACTIMS Meeting, held in September 2020, which highlighted the drug’s mechanisms and compared them with those of other BTK inhibitors. At the end of the presentation, the study authors concluded that fenebrutinib may be safer than less selective, covalent BTK inhibitors and that it has best-in-class potential in MS. Fenebrutinib’s safety profile was further examined and confirmed through an analysis that included data from phase 2 randomized, controlled clinical trials and open-label extensions in diverse autoimmune indications such as rheumatoid arthritis, systemic lupus erythematosus, and chronic spontaneous urticaria. Safety assessments included adverse events (AEs), laboratory results, electrocardiogram results, and vital signs.3
oooooooooooooooo


