Media Contact:
Wendy Ryan/Genevieve Gadenne
Schwartz Communications
781-684-0770
BIONESS INC. RECEIVES FDA CLEARANCE OF ITS NESS L300 PLUS SYSTEM
Device Improves Walking in Stroke Survivors and Individuals with Other Central Nervous System Disorders
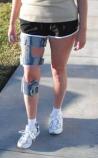
Valencia, Calif. — May 10, 2011 — Bioness Inc. today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its NESS L300® Plus System. The device combines the Company’s NESS L300® Foot Drop System with a thigh stimulation cuff, to provide knee flexion and extension in addition to ankle dorsiflexion during gait. The NESS L300 Plus is intended for persons with upper motor neuron injury or disease resulting from stroke, multiple sclerosis, traumatic brain injury and spinal cord injury.
The device also may facilitate muscle re-education, prevent/retard muscle atrophy, maintain or increase range of motion and increase local blood flow.
People with upper motor neuron injuries or diseases often experience gait movement disorders such as foot drop, which is a result of partial leg paralysis. Gait movement disorders not only result in difficulty walking, but may also lead to fatigue, falls or abnormal walking patterns. The NESS L300 Plus builds on the proven success of Bioness’ NESS L300 Foot Drop System and is designed to additionally stimulate the muscles of the thigh. The addition of the thigh stimulation cuff, synchronized with a wireless heel sensor to detect when the foot is on or off the ground, controls the knee, making it easier to walk. Historically, patients have relied on rigid plastic braces which restrict thigh and ankle movements and can lead to additional problems, including increased falls.
Learn More from : www.bioness.com
===========================================================
Remain CURRENT with Multiple Sclerosis news and information,
.Providing You with ‘MS Views and News’, is what we do
.
On the 4th Wednesday of each month
Each month will feature various guests to be interviewed
Call-in to have (5) minutes of airtime.
Speak about your MS or ask questions
.
Your donation will Help us to educate
“MS Views and News” is a 501©(3) Not-for-Profit organization as recognized by the Internal Revenue Service
.. All contributions are tax deductible to the fullest extent allowed by law
.===========================================================
Disclaimer: ‘MS Views and News’ (MSVN), does not endorse any products or services found on this blog. It is up to you to seek advice from your healthcare provider. The intent of this blog is to provide information on various medical conditions, medications, treatments, and procedures for your personal knowledge and to keep you informed of current health-related issues. It is not intended to be complete or exhaustive, nor is it a substitute for the advice of your physician. Should you or your family members have any specific medical problem, seek medical care promptly.
Visit our MS Learning Channel on YouTube: http://www.youtube.com/msviewsandnews